Abstract
INTRODUCTION:
Gemtuzumab ozogamicin (GO, MylotargTM) was re-approved by the FDA and has been commercially available since September 2017. Prior to re-approval, adults and children with relapsed/refractory AML or APL were treated with GO obtained through an expanded access program under an institutional IND at our Cancer Consortium: Fred Hutchinson Cancer Research Center (FHCRC), the University of Washington Medical Center (UWMC), Seattle Children's Hospital (SCH), and the Seattle Cancer Care Alliance (SCCA).
METHODS:
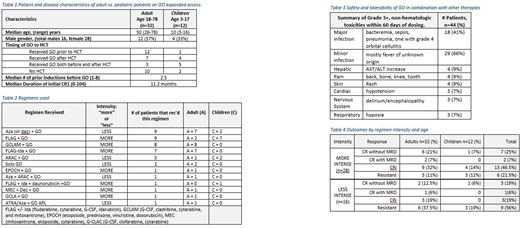
Between 2014-2017, we enrolled 44 patients (median age 40); patient characteristics are summarized in Table 1. Twelve were children treated at SCH and 32 adults at SCCA/UWMC. Forty had AML, 3 mixed phenotype acute leukemia and 1 APL. Forty-two had relapsed disease and 2 were primary refractory. Median duration of CR1 was 11.2 months (range 0-104). Median number of prior induction attempts was 2 (range 1-8).
Eligibility initially required intermediate or "good" risk cytogenetics, though subsequently patients whose blasts were CD33 positive were included regardless of cytogenetics. Additional inclusion criteria were ECOG performance status (PS) ≤3, bilirubin ≤2.0 mg/dl, and ALT/AST ≤5 fold the institutional upper limit of normal.
RESULTS:
Two patients received single-agent GO (9mg/m2 day 1), 1 patient with APL received GO + ATRA + Aza, and the remainder typically received 3mg/m2 with chemotherapy for a single dose or on days 1, 4, and 7 (Table 2). Patients received a median of 2 cycles (range 1-6) of GO (the APL patient received 6 cycles). Timing of GO around hematopoietic stem cell transplant (HCT) is also noted in Table 2.
Table 3 shows the grade 3-4 toxicities occurring within 60 days of receipt of GO per number of patients, rather than number of events. Two children developed sinusoidal obstructive syndrome (SOS) >60 days post GO. Both incidences were post an allogeneic hematopoietic cell transplant (HCT), one at 15 days post HCT and the other 28 days post HCT. The first patient had complete resolution of SOS and remains alive 2 years later, the second patient developed SOS day 28 post HCT then died of an invasive mucormycosis infection in the setting of immunosuppression on day 37 of the HCT. Six deaths occurred within 60 days of beginning GO: 1 from cardiogenic shock, 1 from multiorgan failure post aspiration event, 2 from pneumonia/sepsis, and 2 from disease progression. No deaths were attributed to GO.
Thirteen of the 44 patients (29%; 95% CI:18-44%) achieved complete remission (CR), 3 with measurable residual disease (MRD 7%) (Table 4). The MRD was detected by flow cytometry in 2 patients, and PCR for inversion 16 in 1 patient. Sixteen had CR with incomplete hematologic recovery (CRi) and 15 were resistant or died before assessment. Eight remain alive in CR with a median event free survival (EFS) of 31 months (range 7-47). Thirty-six patients have died, with a median survival of 17.2 months (range 0.5-9.4). Table 4 outlines response by "more" or "less" intense regimens (intensity is defined in Table 2) and by adult vs. pediatric populations.
We found higher response rates in patients who received GO combined with more intensive [78% CR+CRi] rather than less intensive therapy [44% CR+CRi] and in those with fewer prior regimens [100% CR+CRi in patients with 1 prior regimen compared to 48% with ≥2 prior regimens]. In addition, responses were obtained in patients with intermediate risk cytogenetics (11 out of 19 total intermediate = 58%) or unfavorable (3 out of 8 total unfavorable = 37%), although a higher fraction of responses were seen with favorable cytogenetics [t(8;21, inv16, t(15;17)] (15 out of 17 total favorable = 88%).
CONCLUSION:
GO was safe and well tolerated. Based on CR1 duration and number of salvage regimens, the observed 9/28 CR rate with GO + intense therapy compares with a rate of expected 7/28 had the same patients received prior intense salvage therapy without GO (Estey E, Blood [1996/88:756]). GO combinations are a reasonable option for relapsed/refractory AML, but might be of more value in patients with only measurable residual disease.
ACKNOWLEDGEMENTS:
The GO team wishes to acknowledge Pfizer Inc. for their commitment to patients by supplying drug, and our Investigational Pharmacy, the Institutional Review Board, and the FDA for supporting the efforts of this expanded access program in making gemtuzumab ozogamicin accessible to people with relapsed or refractory AML.
Walter:Boehringer Ingelheim Pharma GmbH & Co. KG: Consultancy; Seattle Genetics, Inc.: Consultancy, Other: Clinical trial support, Research Funding; Covagen AG: Consultancy, Other: Clinical trial support, Research Funding; Aptevo Therapeutic: Consultancy, Other: Clinical trial support, Research Funding; Amphivena Therapeutics: Consultancy, Equity Ownership, Other: Clinical trial support, Research Funding; Amgen Inc.: Other: Clinical trial support, Research Funding; Actinium Pharmaceuticals, Inc.: Other: Clinical trial support, Research Funding; Pfizer: Consultancy. Scott:Celgene: Consultancy, Research Funding; Agios: Consultancy; Alexion: Consultancy; Novartis: Research Funding. Cassaday:Adaptive Biotechnologies: Consultancy; Merck: Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Seattle Genetics: Other: Spouse Employment, Research Funding; Jazz Pharmaceuticals: Consultancy; Kite Pharma: Research Funding; Incyte: Research Funding. Becker:GlycoMimetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal